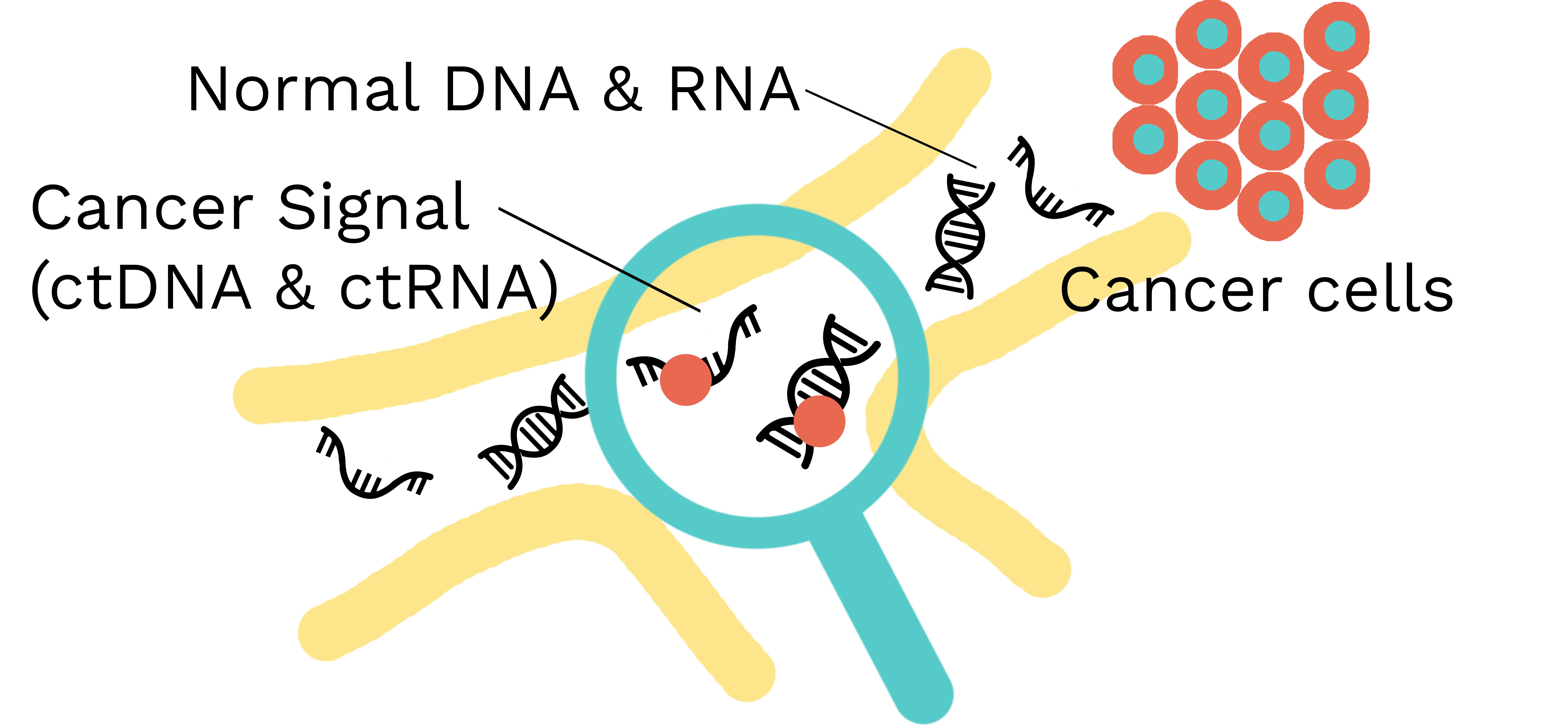
LucenceINSIGHT® is a multi-cancer early detection (MCED) next generation sequencing test that screens for the signal of up to 50 cancers in 1 blood test.
The test uses Lucence’s amplicon-based mirror barcoding technology to detect circulating tumor DNA (ctDNA), circulating tumor RNA (ctRNA) and viral DNA. It then predicts tumor location with machine learning.
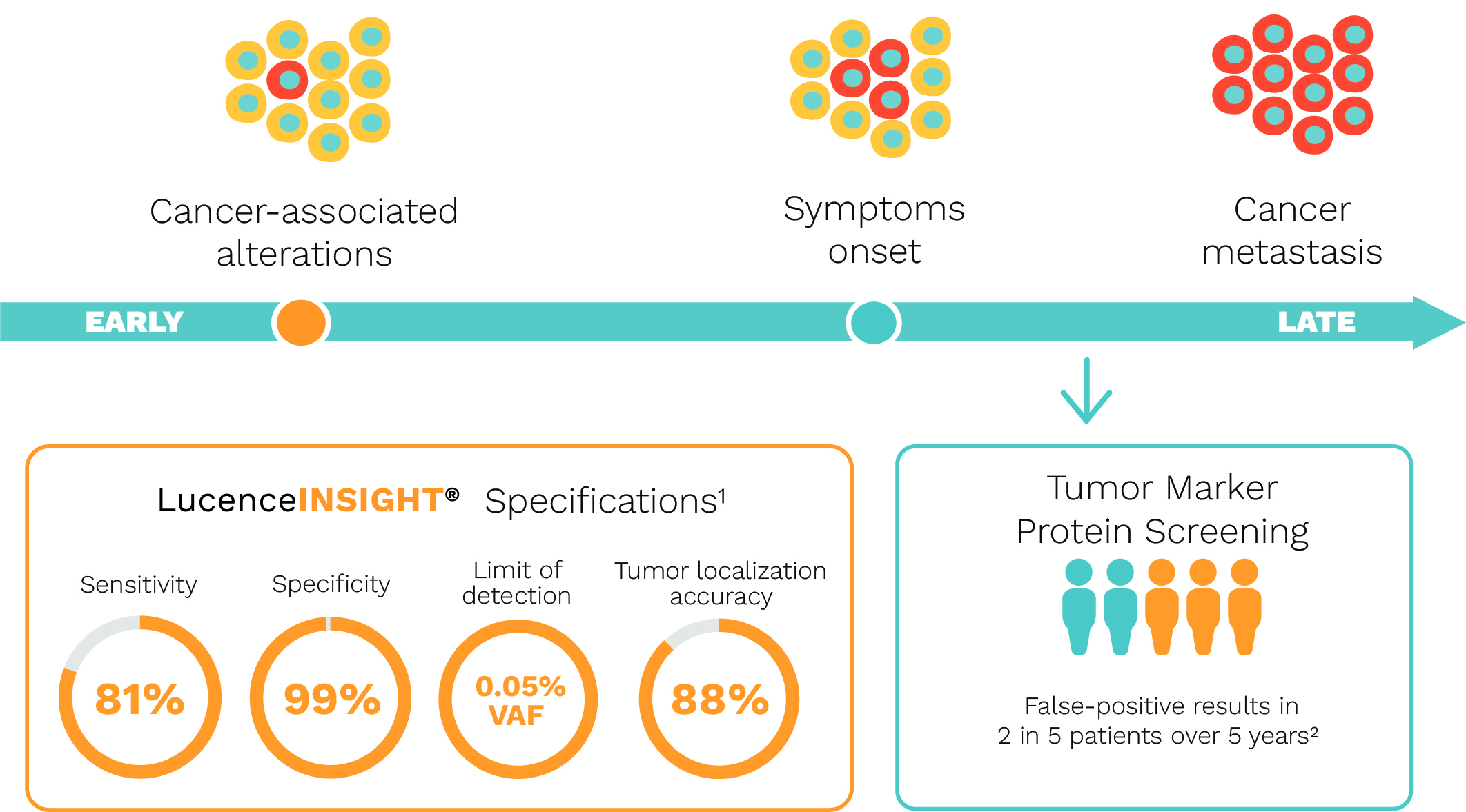
LucenceINSIGHT® Screens For Cancer With High Accuracy.
-
LucenceINSIGHT® reported 100% positive predictive value (PPV) from a prospective real-world cohort of 264 asymptomatic individuals in 2023.3
-
A performance of 81% sensitivity and 99% specificity was reported from a retrospective cohort of 601 patient samples in 2024.1
Boosting Detection with Circulating Tumor RNA (ctRNA) Tumor Markers
ctRNA are cancer signals shed into the blood by living cancer cells.7
The ctRNA tumor marker boost is useful for screening individuals at high-risk:
-
Family history of lung, colon or breast cancer
-
Heavy smokers
ctRNA tumor marker boost is included with LucenceINSIGHT® 50 and available as an optional add-on for LucenceINSIGHT® 5, Women’s 7, and 12.
12.5%
Relative Increase in Sensitivity
for detection of lung, colon and breast cancers with ctRNA + ctDNA*
*Sensitivity increase calculated as relative improvement over a ctDNA-only approach, based on an initial training dataset of three cancer types (breast, lung, colon) and independently validated on an internal dataset. Magnitude of increase varies by cancer type (data on file).4
Case Study Based on LucenceINSIGHT® (ctDNA): Colorectal Cancer3
Frequently Asked Questions
References
- Poh, J. et al. J. Clin. Oncol. 2024; 42(16): e15042.
- Cumulative risk of 43.1% false positives over 14 screening exams from other tumor marker screening methods including chest radiograph, flexible sigmoidoscopy, CA-125, and transvaginal ultrasonography for women, and chest radiograph, flexible sigmoidoscopy, PSA, and DRE for men. (Croswell, J. et al. Ann. Fam. Med. 2009; 7(3): 212–222.)
- Tucker, S. et al. Ann. Oncol. 2023; 34: S1625-S1626.
- Stejskal P, et al. Mol Cancer 2023; 22(15).
©2025 Lucence Diagnostics Pte Ltd. All rights reserved. All logos and trademarks are the property of Lucence or their respective owners. The information on this site is intended for audiences in Singapore and Hong Kong only.

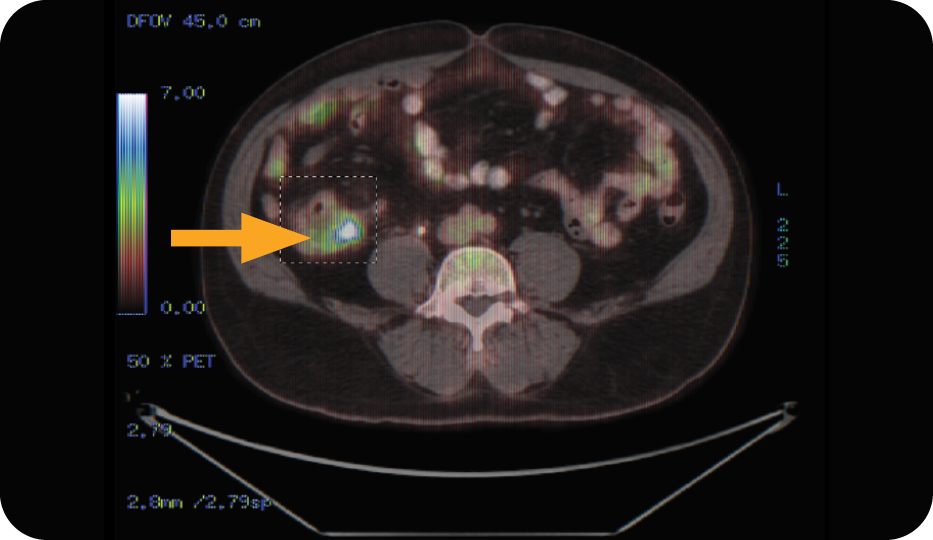
 Focal uptake detected in right ascending colon
Focal uptake detected in right ascending colon