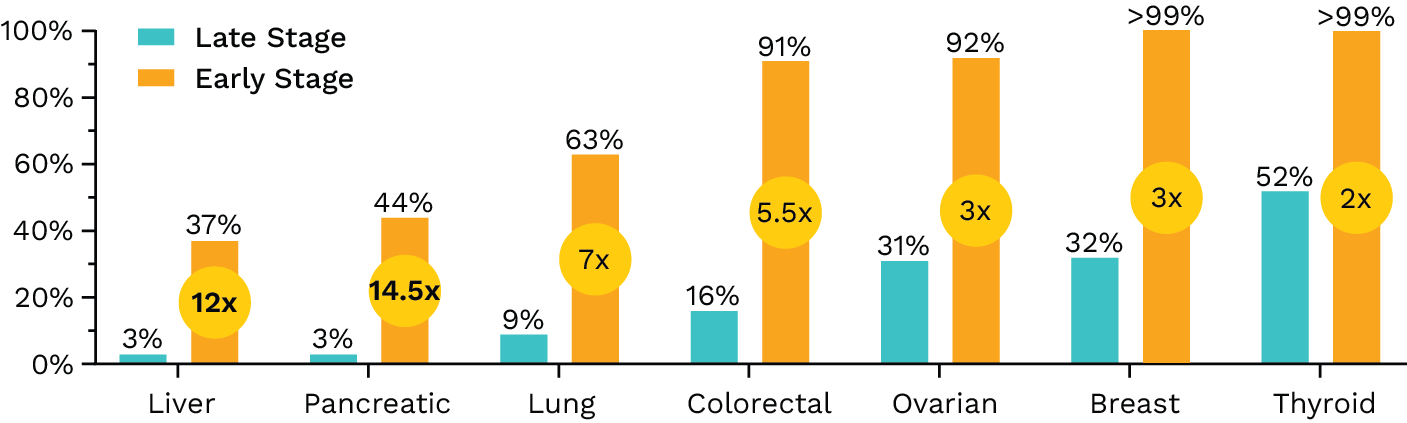
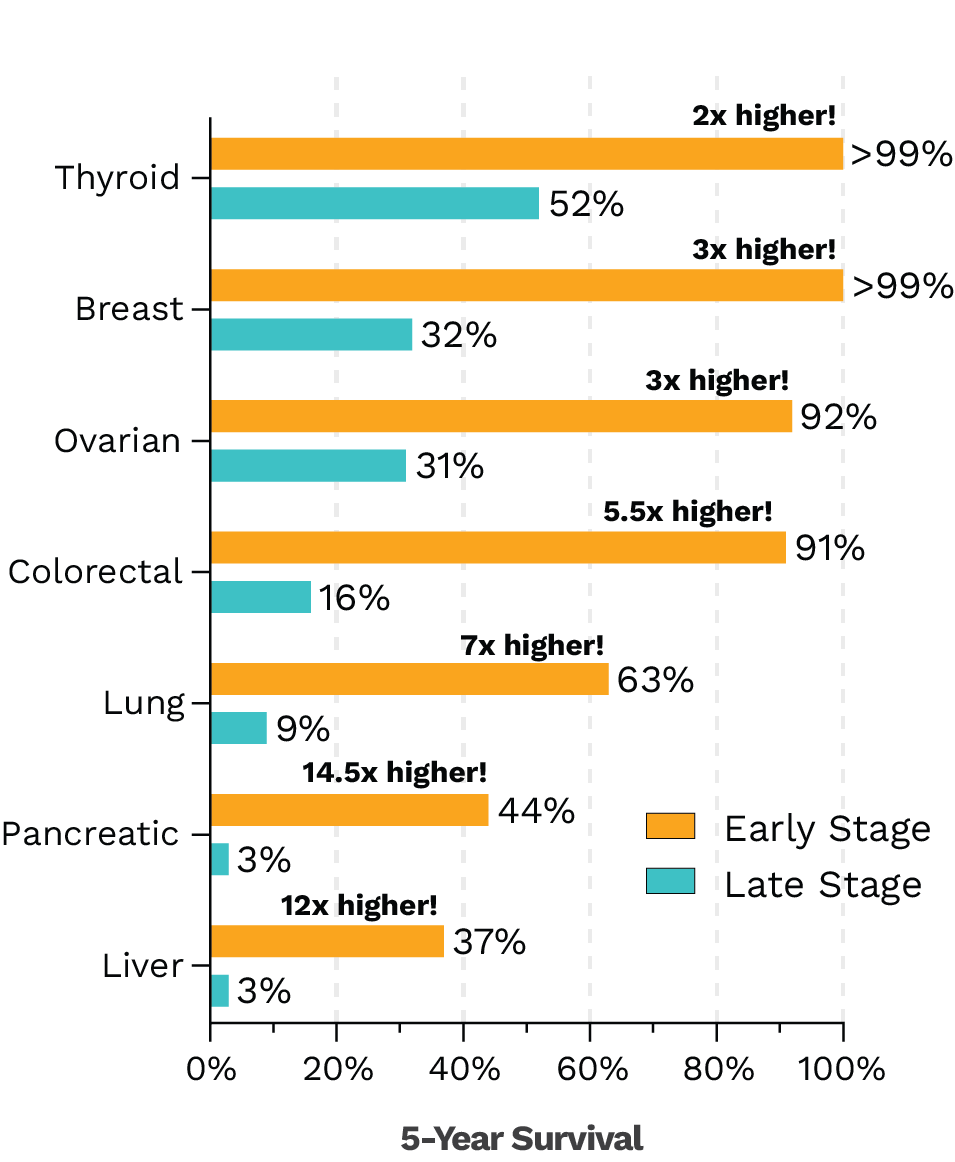
LucenceINSIGHT™ screens for up to 50 cancers with 1 blood draw.
Learn more about multi-cancer early screening.

References
- Statistic derived from data reported in Bray, F. et al. CA Cancer J. Clin. 2024; 74(3): 229-263.
- HealthHub. Screen for Life. Singapore Ministry of Health. https://www.healthhub.sg/programmes/screen_for_life/sfl-faqs#home.
Accessed October 24 2024. - Cancer Research UK. Screening for cancer. https://www.cancerresearchuk.org/about-cancer/screening. Accessed October 24, 2024.
- Hong Kong Cancer Registry. Prevention and screening. https://www.cancer.gov.hk/en/hong_kong_cancer/prevention_and_screening.html#3. Accessed October 24, 2024.
- Centers for Disease Control and Prevention. Cancer screening. https://www.cdc.gov/cancer/prevention/screening.html. Accessed October 24, 2024.
- Statistics derived from data reported in SEER Cancer Stat Facts. https://seer.cancer.gov/statfacts/.
SINGAPORE OFFICE
211 Henderson Rd #04-02, Henderson Industrial Park, Singapore 159552
sales.asean@lucence.com
+65 6592 5102
HONG KONG OFFICE
Rooms 05-15, 13A/F, South Tower World Finance Centre Harbour City,
17 Canton Road, Tsim Sha Tsui, Kowloon Hong Kong
sales.hk@lucence.com
+852 5182 7199
©2025 Lucence Diagnostics Pte Ltd. All rights reserved. All logos and trademarks are the property of Lucence or their respective owners. The information on this site is intended for audiences in Singapore and Hong Kong only.